 When you click on a picture, enlargement accompanied by exploratory text in a new window
When you click on a picture, enlargement accompanied by exploratory text in a new window
- Mechanism and control of plant cell wall loosening: regulation of germination, plant expansion, growth, fruit ripening and abscission
- Enzymic mechanisms, old and new, of wall modification
- XET: a well-known homo-transglycanase activity ubiquitous in plants
- HTG: a novel hetero-transglycanase from horsetails
- Additional novel hemicellulose-remodelling transglycanases, and their evolution from charophytes to angiosperms
- Transglycosidases: novel polysaccharide-modifying enzyme activities that may re-structure the angiosperm primary cell wall
- Characterisation of XTH-activating factor (XAF)
- Non-enzymic mechanisms of wall modification: ascorbate and hydroxyl radicals
- Enzymic mechanisms, old and new, of wall modification
- High-throughput screens for cell wall enzyme activities
- Assembly of the primary cell wall; wall tightening
- Defining the biochemical role of boron in the plant cell wall, and the basis of boron toxicity
- The role of peroxidase-catalysed oxidative phenolic coupling and other cross-linking reactions in wall tightening
- Sugar nucleotide metabolism
- Oligosaccharins (biologically-active oligosaccharides)
- Evolution of primary cell wall in lower land plants
- Biomaterials
1. Mechanism and control of plant cell wall loosening: regulation of germination, plant expansion, growth, fruit ripening and abscission
Changes in the mechanical properties of primary cell walls ('loosening' and 'tightening') are evoked during plant development; some are triggered by auxins, gibberellins, ethylene and other hormones. These changes play a major role in governing the rate and pattern of plant growth. The Edinburgh Cell Wall Group is investigating the chemical basis of these physical changes in the cell wall. Some of the changes are due to enzyme action, whereas others have a non-enzymic basis.
In 1989, we discovered an interesting enzyme activity, later named xyloglucan endotransglucosylase (XET), which breaks and re-forms glycosidic bonds in the backbone of xyloglucan, a major structural polysaccharide of primary cell walls. XET is one of two activities exhibited by XTH proteins, the other being XEH .
- XET = xyloglucan endotransglucosylase (activity)
- XEH = xyloglucan endohydrolase (activity)
- XTH = xyloglucan endotransglucosylase/hydrolase (protein)
|
|
✚ |
|
―(XET)→ |
|
✚ |
|
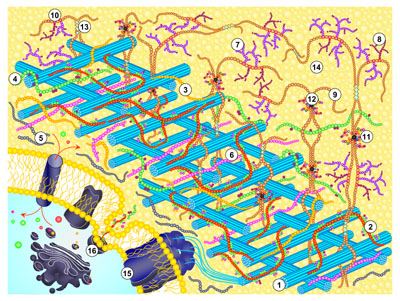
where ❍❍❍... is a xyloglucan chain serving as donor substrate (⦿ is its reducing end), and ❂❂❂.. is another xyloglucan chain serving as acceptor substrate. The donor and acceptor can be chemically identical. This type of reaction has been described as ‘cutting and pasting’ of polysaccharide chains. Its biological role may be to integrate newly synthesised xyloglucan chains into the cell wall and/or to re-structure the old xyloglucan chains that are already present, thus loosening the wall, for example - as shown in the diagram:
|
XTH proteins belong to CAZy family GH16. XET activity is now widely regarded as a prime candidate for playing a key role in wall loosening during cell expansion and fruit ripening. The properties of this enzyme, and its role in reversibly loosening the wall, are under intensive investigation. Following the discovery of XET activity, molecular biological studies have led to the discovery of 33 different XTH genes in Arabidopsis. The Edinburgh Cell Wall Group is interested in understanding the enzymological properties of these XET-active isoenzymes and in monitoring qualitatively and quantitatively the reactions catalysed by XET activity in the walls of living plant cells. The latter undertaking is a particular challenge because the substrates of XET activity are often chemically indistinguishable from the products; our approaches include the use of 13C/2H/3H-pulse-chase experiments so that we can detect XET action by physical changes (in buoyant density). XET action can be visualised in plant tissues if trace levels of a small, fluorescently or radioactively labelled acceptor substrate (✪) is supplied to the tissue. The reaction is basically as above, but modified as follows:
|
|
✚ | ✪ | ―(XET)→ |
|
✚ |
|
The donor substrate and the enzyme are endogenous to the tissue. One of the products (❍❍❍❍❍❍❍✪) is easily detected owing to its label, as shown here:
|
|
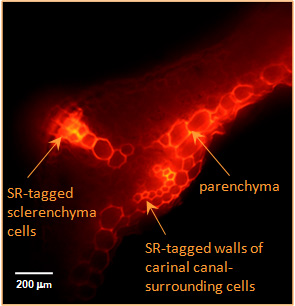
| Transglycanase action detected in the cell walls of living plant organs. Both the enzyme (XTH or HTG) and the donor substrate (xyloglucan and/or MLG) are endogenous to the organs. A small, fluorescently labelled acceptor substrate (xyloglucan-oligosaccharide–sulphorhodamine) was supplied exogenously. Fluorescence indicates the location of transglycanase action, creating polysaccharide–sulphorhodamine conjugates. |
| Left: XET action in the elongation zone of an Arabidopsis root. Root tip (non-fluorescent) is at the bottom. | Right: XET and MXE action in Equisetum fluviatile tissue. |
HTG (hetero-trans-β-glucanase) is a unique new enzyme recently discovered in Equisetum plants (horsetails). One of the activities exhibited by this enzyme was named mixed-linkage β-glucan : xyloglucan endotransglucosylase (MXE). Remarkably, this is a hetero-transglycanase — i.e., its preferred donor substrate, MLG [mixed-linkage (1→3),(1→4)-β-glucan], is qualitatively different from its preferred acceptor substrate (xyloglucan). HTG appears not to be found in any other land-plants than Equisetum, which is feasible since Equisetum is probably the most evolutionarily isolated genus of all land-plants, its closest living relatives having diverged from it >370,000,000 years ago. Evidently some time during the Upper Devonian and now, these plants ‘invented’ HTG. Initially, we were very surprised to discover in Equisetum an enzyme that targets MLG, because this polysaccharide was hitherto known only from members of the Poales (including grasses and cereals); however, looking for an MXE substrate in Equisetum led us to discover that MLG is not confined to the Poales but also occurs in Equisetum (though no other fern-allies ). Since the Poales possess MLG but not HTG, the exciting possibility exists of introducing this enzyme into important cereal crops.
Although within the land-plants MXE activity is unique to the genus Equisetum, we did also find MXE activity in charophytic algae — the closest algal relatives of land-plants. We have also detected several other transglycanase activities in charophytes, including the recently discovered trans-β-xylanase (xylan endotransglycosylase) (Franková & Fry, unpublished). As a background to our enzyme survey, we are also monitoring the evolution of the plant cell wall from algae to land plants (O’Rourke & Fry, unpublished). It has often been noted that major evolutionary steps in the plant kingdom were accompanied by significant changes in cell-wall chemistry.
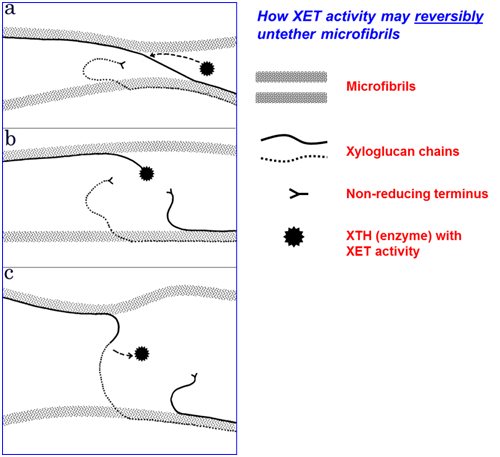
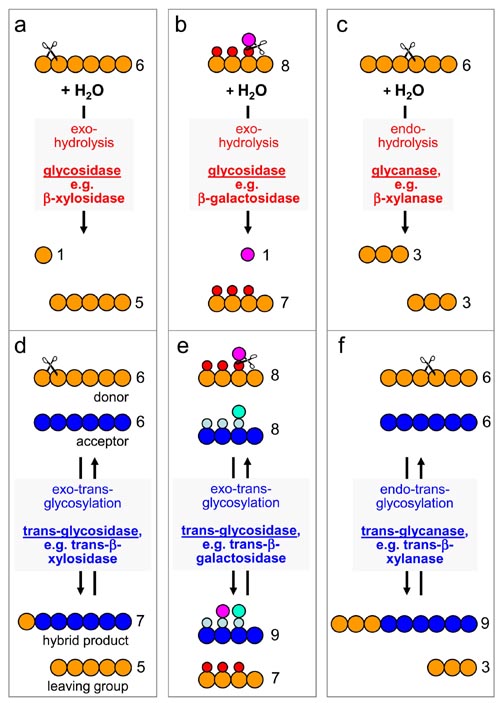
In addition to transglycanases, plant cell walls have recently been found to possess several transglycosidase activities. Transglycanases are endo-enzymes, cleaving and re-forming internal glycosidic bonds of a polysaccharide backbone; transglycosidases in contrast are exo-enzymes, i.e. they cleave and re-form non-reducing terminal glycosidic bonds. This distinction, and these enzyme activities’ correspondence with comparable hydrolases, are summarised in the diagram below [figures a-f].
|
|
|
Examples of transglycosidase activities that can act on wall polysaccharides include
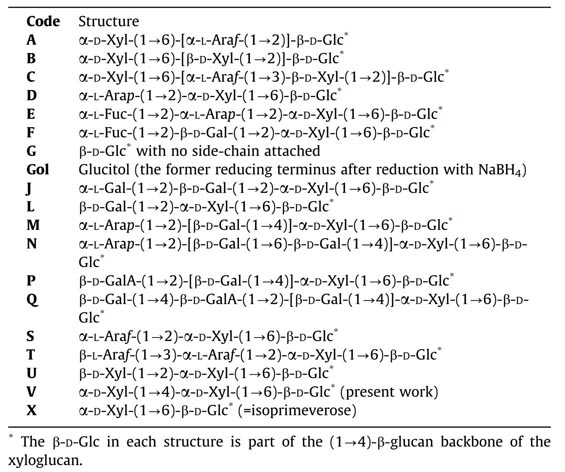
- Trans-α-xylosidase, an activity that transfers a xylose residue from one xyloglucan chain to another, creating a novel xyloglucan building-block, α-D-Xylp-(1→4)-α-D-Xylp-(1→6)-D-Glc (abbreviation: ‘V’) [figures above].
- Trans-β-galactosidase, an activity that transfers a galactose residue from one xyloglucan chain to another (the product remains to be characterised in detail).
- Trans-β-xylosidase, an activity that transfers a xylose residue from one xylan chain to another.
The action of plant cell wall enzymes can be modulated in vivo to suit the current needs of the plant (cell). We found that plants contain heat-stable polysaccharide-like substances that appear to ‘activate’ XTHs, increasing XET activity. We are currently further characterising this XTH-activating factor (XAF), and its probable role in the control of plant growth and development. It now appears that XAF mainly activates XTHs by releasing them from a tightly wall-bound state (Cam Tu, Sharples and Fry, unpublished).
|
Plant cell walls contain not only structural polysaccharides and glycoproteins and phenolics, but also diverse low-molecular-weight solutes (dissolved in the apoplast — the aqueous solution that permeates the walls of living plant cells). An interesting example is ascorbate (vitamin C). Most of the vitamin C in a plant is intra-protoplasmic, but a significant minority is apoplastic. The apoplastic vitamin C is of interest because it tends to break down in vivo, a process that may be a major source of vitamin loss. Studying this breakdown is important for two main reasons:
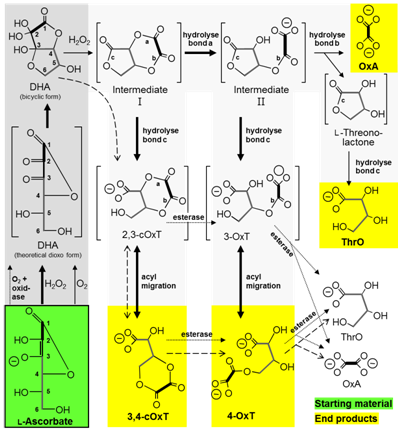
(a) The vitamin C content of plant tissue is partly dictated by vitamin breakdown (in opposition to vitamin synthesis). Manipulating the breakdown pathway may be a means of enhancing the vitamin C content of fruit and veg. Until recently, little was known about ascorbate breakdown pathways. We mapped the pathways in outline , demonstrating the intermediacy of several hitherto unknown intermediates including 4-O-oxalyl-L-threonate. The pathway was later refined [diagram on left], and current work is continuing to monitor the losses of vitamin C that occur during storage of harvested salad plants (Dewhirst & Fry, unpublished).
(b) Apoplastic ascorbate is an important recognised source of hydroxyl radicals (•OH), which are capable of cleaving wall polysaccharide.
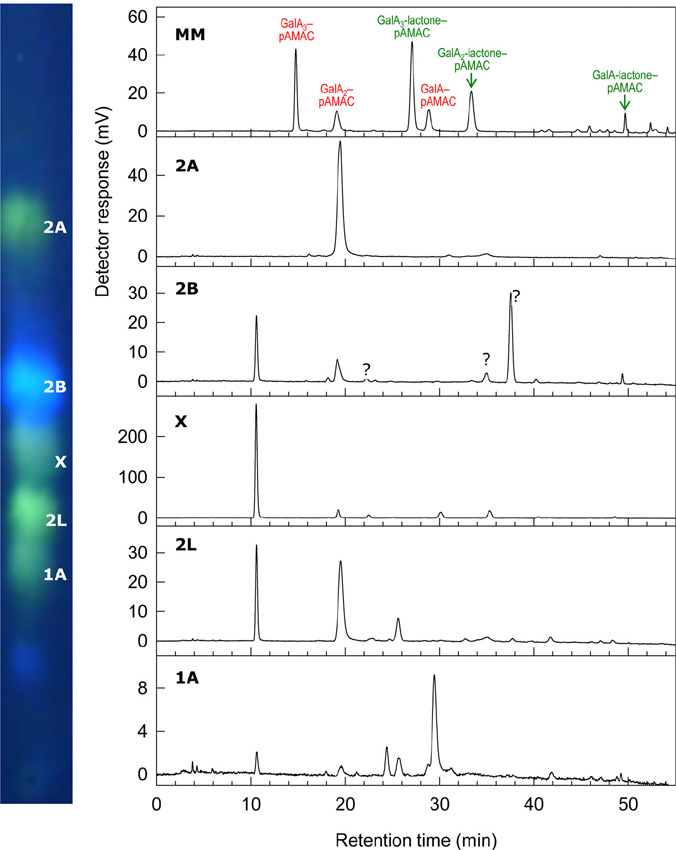
|
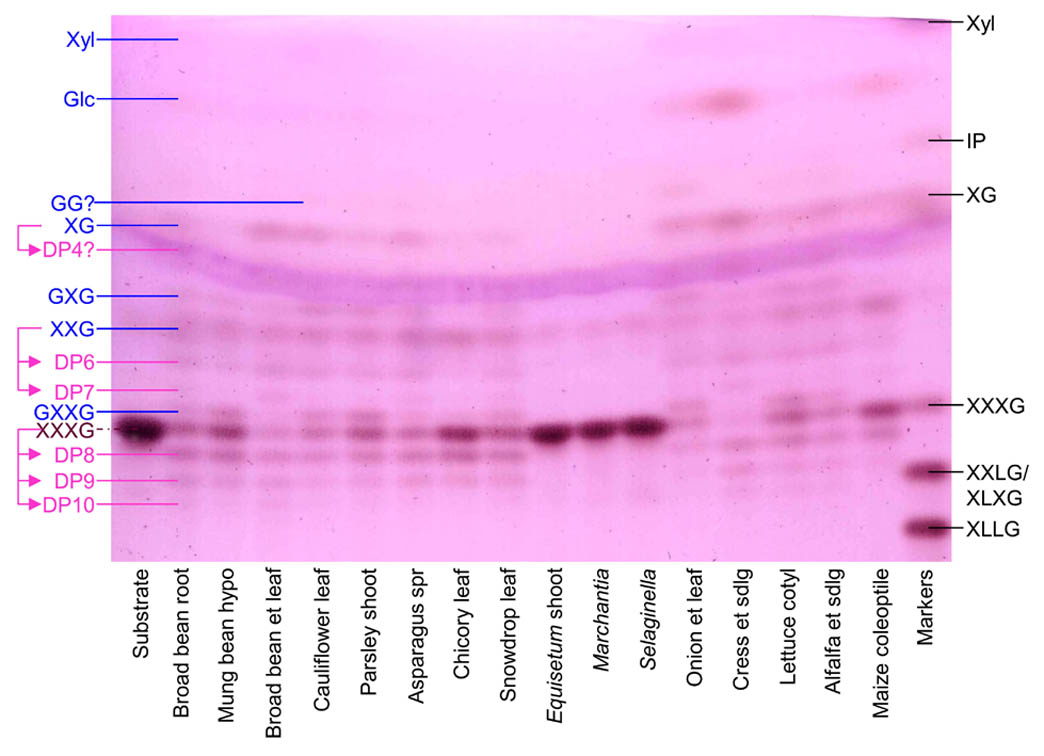
The hydroxyl radical (•OH) is the most reactive known molecule. It can be generated very readily in the presence of ascorbate, O2 and minute traces of a transition metal (e.g. Cu2+ or Fe3+). These three agents occur together in the apoplast (aqueous solution that permeates the walls of living plant cells). The •OH radical can cleave polysaccharides, potentially loosening the cell wall and enhancing tissue softening, e.g. in ripening fruit. Since •OH radicals are exceedingly short-lived in a biological milieu, they are difficult to document. We have developed membrane-impermeant probes with which •OH can be detected and quantified. In addition, we have developed methods to demonstrate the presence of the particular type of ‘collateral damage’ (introduction of mid-chain C=O oxo groups) suffered by polysaccharides when they are cleaved by •OH. Of two methods developed, one uses radiolabelling to detect the mid-chain C=O groups, and the other uses a fluorescent tag. Cell wall polysaccharides ‘labelled’ by either of these methods can then be digested (e.g. with Driselase) to easily detected, low-molecular-weight products [oligosaccharides; figure on left].
2. High-throughput screens for cell wall enzyme activities
We are developing high-throughput screens for hydrolase and transglycosylase activities and for their inhibitors. This work is helping to define the range of enzyme activities that occur in different plants throughout the plant kingdom. Discovering ‘xenobiotics’ that act as inhibitors of cell-wall enzymes can be valuable for enabling future experiments to use 'chemical genetics' to define the biological role of cell wall-acting enzymes, for example in cell expansion. Inhibitors that target essential wall enzymes are also potential starting points for the development of novel herbicides. Therefore we are in the process of defining the effectiveness and in-vivo phytotoxicity of selected 'hit' xenobiotics on model monocots and dicots. We are also continuing to screen diverse xenobiotics for their ability to inhibit cell-wall hydrolases and transglycosylases. To date we have developed high-throughput screens for numerous cell-wall enzyme activities, including:
- pectin methylesterase
- XET
- MXE
- β-(1→4)-glucosidase
- β-(1→3)-glucosidase
- β-xylosidase
- β-galactosidase
- β-mannosidase
- α-xylosidase (xyloglucan-acting)
- α-fucosidase (xyloglucan-acting)
- α-galacturonidase
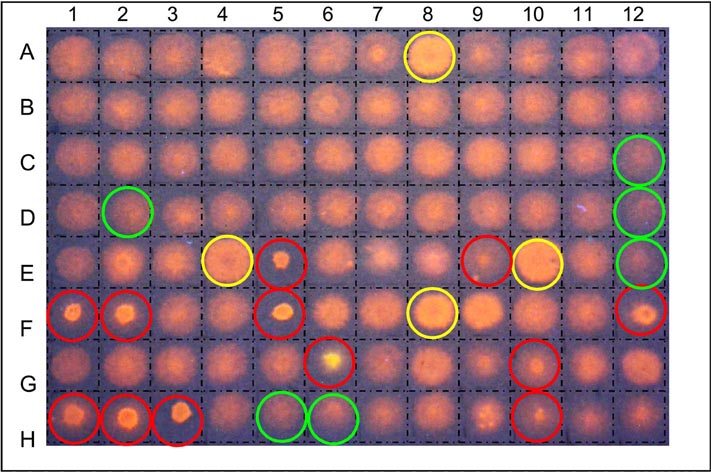
|
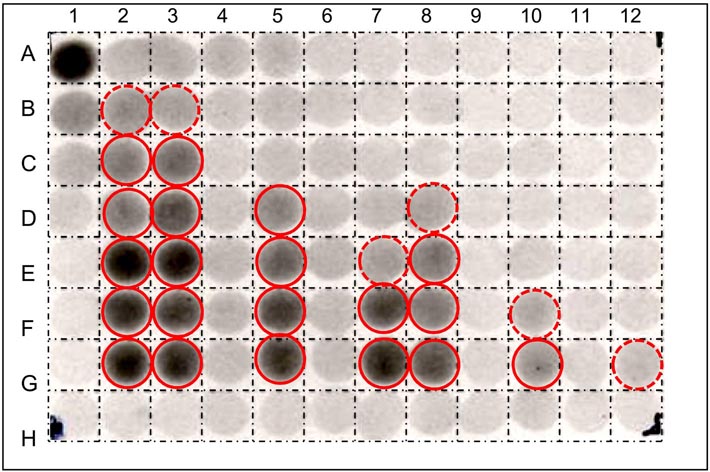
|
| High-throughput screens for XET inhibitors on test strata impregnated with polysaccharide donor followed by fluorescently-tagged oligosaccharide acceptor (Franková & Fry, unpublished) | High-throughput screens for β-mannosidase inhibitors using radiolabelled substrate - [3H]Man6-ol (Chormova & Fry, unpublished) |
Many of these assays involve the use of ‘dot-blot’ test papers which have been impregnated with a suitable radioactively or fluorescently labelled substrate — the labelled reaction products formed by the enzyme are then washed out of the paper either more or less easily than the original labelled substrate [image of XET test paper dot-blot picture on far left]. This allows us to search either (a) for the presence of the activity (e.g. XET) in diverse plant extracts or column fractions, or (b) for the presence of inhibitors among a collection of xenobiotic compounds mixed with a standard XET enzyme prep. Alternatively, the labelled substrate is incubated for a given time with 5 µl of the enzyme solution (± added xenobiotics), then spotted onto plain filter paper, and washed in a suitable solvent, e.g. 75% ethanol. In the case of β-mannosidase, the substrate is [3H]Man6-ol (radiolabelled hexasaccharide), and the enzyme creates smaller oligosaccharides such as [3H]Man2-ol. The former remains within the paper during the washing step, whereas the latter is washed out, leaving a non-radioactive zone [image of [3H]Man6-ol dot-blot on left].
3. Assembly of the primary cell wall; wall tightening
Unlike most organisms, plants have a readily demonstrable requirement for boron (B), which is available to plants as soluble boric acid, H3BO3 (pKa 9.1). This agriculturally important feature of plant life is poorly understood biochemically. Despite being an essential element, excess B is detrimental to plants, and there is a narrow window between concentrations giving deficiency and those producing toxicity. Problems of B deficiency can be solved with fertilisers, but excess B is an intractable agricultural problem, especially in some arid areas. Understanding why plants require B, and the basis of its toxicity, will facilitate progress in agriculture, and is an aim of our laboratory.
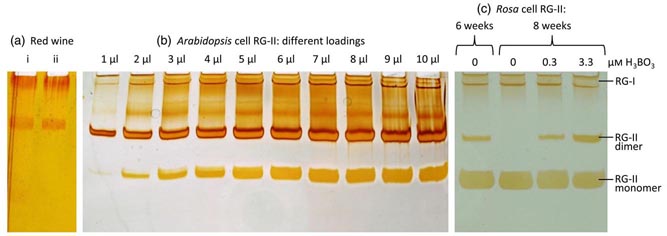
|
The cell-wall pectic domain rhamnogalacturonan-II (RG-II) is cross-linked via borate diester bridges, which influence the expansion, thickness and porosity of the wall. Until recently, little was known about the mechanism or subcellular site of this cross-linking. Using polyacrylamide gel electrophoresis (PAGE) to separate monomeric from dimeric (boron-bridged) RG-II, we studied the dimerisation process in vitro. For in-vivo experiments, we successfully cultured ‘Paul’s Scarlet’ rose (Rosa sp.) cells in boron-free medium: their wall-bound pectin contained monomeric RG-II domains but no detectable dimers. We conclude that pectins containing RG-II domains can be held in the wall other than via boron bridges.
Re-addition of H3BO3 to 3.3 µM triggered a gradual appearance of RG-II dimer over 24 h but without detectable loss of existing monomers, suggesting that only newly synthesised RG-II was amenable to boron bridging. In agreement with this, Rosa cultures whose polysaccharide biosynthetic machinery had been compromised (by carbon starvation, respiratory inhibitors, anaerobiosis, freezing or boiling) lost the ability to generate RG-II dimers. We conclude that RG-II normally becomes boron-bridged during synthesis or secretion but not post-secretion. Supporting this conclusion, exogenous [3H]RG-II was neither dimerised in the medium nor cross-linked to existing wall-associated RG-II domains when added to Rosa cultures. In conclusion, in cultured Rosa cells RG-II domains have a brief window of opportunity for boron-bridging intraprotoplasmically or during secretion, but secretion into the apoplast is a point of no return beyond which additional boron-bridging does not readily occur.
Compared with its role in cross-linking the pectic domain rhamnogalacturonan-II (RG-II), little information is known about the biological role of B in membranes. We are interested in the involvement of glycosylinositol phosphorylceramides (GIPCs), major components of lipid rafts, in the membrane requirement for B. Using thin-layer chromatography and mass spectrometry, we have characterised GIPCs from Rosa cell culture. Disrupting B bridging (by B starvation in vivo or by treatment with cold dilute HCl or with excess borate in vitro) enhanced the GIPCs' extractability. As RG-II is the main B-binding site in plants, we investigated whether it could form a B-centred complex with GIPCs. Using high-voltage paper electrophoresis, we showed that addition of GIPCs decreased the electrophoretic mobility of radiolabelled RG-II, suggesting formation of a GIPC–B–RG-II complex (figure below).
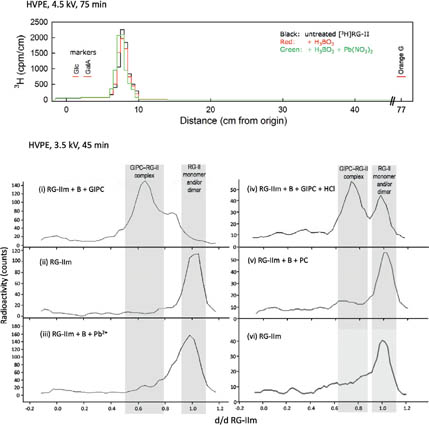
|
Last, using polyacrylamide gel electrophoresis, we showed that added GIPCs facilitate RG-II dimerization in vitro. We conclude that B plays a structural role in the plasma membrane. The disruption of membrane components by high borate may account for the phytotoxicity of excess B. Moreover, the in-vitro formation of a GIPC–B–RG-II complex gives the first molecular explanation of the wall–membrane attachment sites observed in vivo. Finally, our results suggest a role for GIPCs in the RG-II dimerization process. As RG-II is the main B-binding site in plants, we investigated whether it could form a B-centred complex with GIPCs.
Work on covalent cross-links is based on the hypothesis that some or all of the following bonds contribute to the coherency of the primary cell wall:
- oxidative coupling-products formed between pairs of phenolic side-chains of cell wall polymers by the action of peroxidases and laccases (e.g. dimers, trimers and tetramers of tyrosine residues in glycoproteins, and diferulate and novel relatives thereof formed from the feruloyl groups of polysaccharides);
- O-uronoyl ester bonds formed between the -COOH groups of pectic polysaccharides and the -OH groups of other wall polymers;
- N-uronoyl amide bonds formed between the -COOH groups of pectic polysaccharides and, for example, the ε-NH2 groups of lysine residues in wall glycoproteins.
For the discovery of novel cross-links, our general approach for each putative covalent cross-link is to synthesise chemically a range of model compounds of the type under investigation, characterise these (especially with respect to their chromatographic properties and resistance to chemical and enzymic treatments), and then to look for the occurrence of these and related substances in enzymic digests of plant cell walls. The chemical characterisation of these novel (synthetic and natural) compounds has recently been promoted by 2-dimensional NMR analysis made possible by a productive collaboration with Dr I.H. Sadler, Dept of Chemistry.
4. Sugar nucleotide metabolism
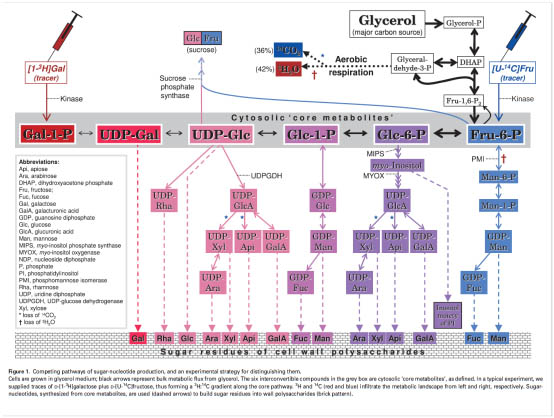
|
The Edinburgh Cell Wall Group has developed a method for studying the in vivo inter-conversion of the precursors of wall polysaccharide biosynthesis. There are several examples where two or more putative pathways lead to the same important metabolite (e.g. UDP-glucuronate and GDP-mannose); we are employing techniques of in-vivo dual-isotope radiolabelling to dissect which of these apparently competing pathways predominate in the living cell [figure left]. Attempts to solve this problem by use of gene knock-outs are confused by the remarkable ability of plant cells to compensate for the lack of one pathway by exploiting an alternative pathway — one which does not necessarily occur to any appreciable extent in the normal cell.
5. Oligosaccharins (biologically-active oligosaccharides)
Specific oligosaccharides, produced from cell wall polysaccharides such as pectins and xyloglucan, are known to exert diverse and potent physiological effects on living plant cells. Such oligosaccharides are termed oligosaccharins. Our work in this area focuses on the synthesis, degradation and transport of oligosaccharins in vivo as well as their biological effects. Biosynthetic studies have revealed the natural production of a novel oligosaccharin that appears to be derived from glycosphingolipids, a poorly understood group of glycolipids. This has opened an interest in these particular glycolipids (GIPCs) - their occurrence, structure and biological significance [The Plant Journal 79: 139–149, 2014].
We are also currently exploring the in-vivo production and role of lepidimoide, a rhamnogalacturonan-I-related disaccharide that has been reported to affect seedling growth, and is claimed to be an allelochemical.
6. Evolution of primary cell wall in lower land plants
Work has begun to trace the changes in primary cell wall composition that have occurred during the evolution of land plants (embryophytes) from their hypothetical algal ancestors (charophytes) and during the diversification of land plants from bryophyte-like ancestors. Our results show that major events in plant evolution were often accompanied by remarkable changes in cell wall chemistry, indicating a special role for cell wall modification in adapting plants to their newly colonised environments.
Our more recent work is supplying a picture of charophyte cell-wall composition in which the polysaccharides show similarities to, but also many differences from, the corresponding land-plant polymers. Thus, dramatic cell-wall changes occurred during terrestrialisation. Analysing representative charophytes (Chlorokybus, Klebsormidium, Nitella, Chara, Coleochaet), we found that most charophytes, in common with land-plants, possess pectic homogalacturonan (except Klebsormidium) and hemicellulosic xylans. Unlike most land-plants, Chara and Nitella have abundant mannans, though Coleochaete and Chlorokybus do not. Few land-plants (grasses and horsetails) possess mixed-linkage β-glucan (MLG); likewise, most charophytes lack MLG; however, Coleochaete has traces of an interesting MLG-like polysaccharide that differs from land-plant MLG in containing both xylose and glucose. No charophytes contained detectable xyloglucan; in this respect they differ radically from all land-plants.
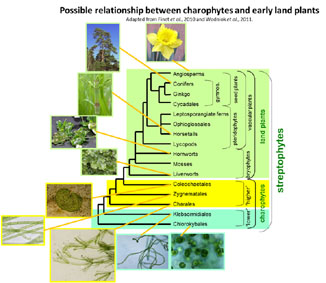
|
Of all charophyte walls analysed, Coleochaete most closely resembles land-plants: it possesses cellulose; pectins rich in galacturonic acid, rhamnose, galactose and arabinose; heteroxylans; negligible mannans; and traces of MLG-like polysaccharide; but lacks xyloglucan.
Despite the chemical chasm between charophyte and embryophyte walls, the functional differences are smaller. We devised novel methods for discovering/measuring wall-remodelling enzymes (transglycanases), and found that many charophytic transglycanases act smoothly on land-plant polysaccharides, suggesting that fundamental biological processes occurring in cell walls (enabling cell expansion etc.) were conserved during the dramatic evolutionary leap from water to land 460,000,000 years ago. Charophytes possess xyloglucan- and MLG-remodelling enzymes, and six other so-far unreported enzyme activities capable of functioning on land-plant polysaccharides. Despite the absence of xyloglucan and paucity of MLG in charophytes, these polysaccharides serve as good substrates for charophytic enzymes. Trans-β-mannanase and trans-β-xylanase may be charophytic alternatives to xyloglucan endotransglucosylase (XET), which prevails in land-plants.
7. Biomaterials
In a new collaboration with CelluComp, Fife, we are working on a project in an interesting combination of Academia (plant cell-wall polysaccharide biochemistry) and the Biotechnology Industry (cellulose/resin biocomposites). The biocomposites will exploit major agricultural ‘waste’ materials (e.g. sugarbeet pulp, down¬stream of the sugar industry).
CelluComp Ltd have patents on the preparation of commercially useable cell-wall fibrils from ‘waste’ biomaterials. The isolated fibrils can be combined with epoxy resins etc. to form biocomposites. Likely commercial uses for the cellulose/resin biocomposites include automotive parts, sports goods, wind-turbine components, paints and coatings.
Currently there is some variability in cellulose/resin interactions, probably due to variation in (i) the presence of residual non-cellulosic wall polysaccharides and (ii) the accessibility of -OH groups on microfibril surfaces. We are exploring the chemical basis of this variability by analysing cell walls from inedible parts of crop-plants, and identifying chemical characteristics that correlate with successful cellulose/resin bonding.

